electron configuration for aluminum|full electron configuration for chlorine : Bacolod How to Write the Electron Configuration for Carbon. Carbon is the sixth element with .
Converting New York Time to GMT. This time zone converter lets you visually and very quickly convert New York, New York time to GMT and vice-versa. Simply mouse over the colored hour-tiles and glance at the hours selected by the column. and done! GMT is known as Greenwich Mean Time. GMT is 4 hours ahead of New York, New York time.
PH0 · what is sodium complete electron configuration
PH1 · how to write electron configuration
PH2 · ground state electron configuration chart
PH3 · give the full electron configuration for sulfur
PH4 · full electron configuration for chlorine
PH5 · electron configuration list
PH6 · electron configuration chart
PH7 · electron configuration calculator
PH8 · Iba pa
Dear BulSU Aspirants: Bulacan State University is pleased to open its Online Application for incoming freshmen for Academic Year 2024-2025. Please be informed that interested applicants may access.
electron configuration for aluminum*******In order to write the Aluminium electron configuration we first need to know the number of electrons for the Al atom (there are 13 electrons). When we write the configuration we'll put all 13 electrons in orbitals around the nucleus of the Aluminium atom.In order to write the Sulfur electron configuration we first need to know the .In order to write the Silicon electron configuration we first need to know the .
In order to write the Argon electron configuration we first need to know the .
When we write the configuration we'll put all 15 electrons in orbitals around the .How to Write the Electron Configuration for Carbon. Carbon is the sixth element with .How to Write the Electron Configuration for Oxygen. Oxygen is the eighth element .
When we write the configuration we'll put all 19 electrons in orbitals around the .In order to write the Mg electron configuration we first need to know the .Learn the electron configuration of aluminum, a non-ferromagnetic metal with atomic number 13 and symbol Al. See how its electrons are distributed in different energy levels and orbitals, and its properties and isotopes. Mar 23, 2023 Electron Configuration of Aluminum. To find the electron configuration of an atom, you first need to know the number of electrons that it has. Since aluminum's . A step-by-step description of how to write the electron configuration for Aluminum (Al). In order to write the Al electron configuration we first need to kn.The electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and subshells. Commonly, the electron configuration is used to . The arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. We describe an electron configuration with a .Elements. Aluminium (Al) Aluminium is a chemical element of the periodic table with chemical symbol Al and atomic number 13 with an atomic weight of 26.9815 u and is .
Electron configuration The arrangements of electrons above the last (closed shell) noble gas. Melting point The temperature at which the solid–liquid phase change occurs. .
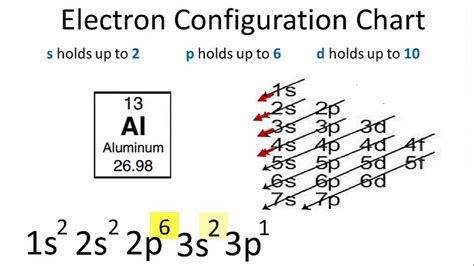
1 Answer. Aluminium has atomic number 13 so the full electron configuration will be: 13Al 1s22s22p63s23p1. A shorthand way of writing this is to use the preceding noble gas configuration by putting its symbol in square brackets in front of the valence electrons. In this case that is neon which is: The electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2. The helium atom contains two protons and two electrons. The first electron has the same four quantum numbers as the hydrogen atom electron ( n = 1, l = 0, ml = 0, ms = +1 2 m s = + 1 2 ). The first two subshells of the third shell are filled in order—for example, the electron configuration of aluminum, with 13 electrons, is 1s 2 2s 2 2p 6 3s 2 3p 1. However, a curious thing happens after the 3p subshell is filled: the 4s subshell begins to fill before the 3d subshell does. In fact, the exact ordering of subshells becomes more .Electron configuration The arrangements of electrons above the last (closed shell) noble gas. Melting point . Aluminium is used in a huge variety of products including cans, foils, kitchen utensils, window frames, beer kegs and aeroplane parts. This is because of its particular properties. It has low density, is non-toxic, has a high thermal .
The atomic number of aluminum is 13. A neutral atom of aluminum has 13 protons and 13 electrons. The ground state electron configuration for aluminum is 1s22s22p63s23p1. A shorthand way to write the electron configuration, called noble gas notation, is [Ne]323p1. An atom in its lowest energy state is said to be in its ground state.
The aluminium atom contains 13 electrons. The electronic configuration of the aluminium is expressed in the form of the diagram as given below-. 1s orbital having minimum energy is filled first, with a maximum capacity of two electrons. After 1s orbital, the 2s orbital is filled with a maximum capacity of two electrons.
Q. Aluminum (Al) atom has ground-state electron configuration as : Q. What is electronic configuration .How to find the electronic configuration of metals as a ex.oxygen. Q. What are the valencies of the element given below. carbon. electronic configuration. 2,4. magnesium. electronic configuration. 2,8,2. oxygen. electronic . Introduction to electron configurations. Electron configurations describe where electrons are located around the nucleus of an atom. For example, the electron configuration of .Electron Configurations. The electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and subshells. Commonly, the electron configuration is used to describe the orbitals of an atom in its ground state, but it can also be used to represent an atom that has ionized into a cation .
Let's find the ground state electron configuration of Aluminum! A single Aluminum atom has 13 protons and 13 electrons, but how do we know where Aluminum put.
Summary. Aluminum is a chemical element. Its symbol is Al. it is almost impossible to find pure aluminum in nature because it is such a highly reactive element. Aluminum is not a magnetic material. . Aluminium is a chemical element with atomic number 13 which means there are 13 protons and 13 electrons in the atomic structure. The chemical symbol for Aluminium is Al. Electron Configuration and Oxidation States of Aluminium. Electron configuration of Aluminium is [Ne] 3s2 3p1. Possible oxidation states are -2; -1; +1; +2; .
The electron configuration for aluminum is 1s2, 2s2, 2p6, 3s2, 3p1. This is assuming the aluminum atom is a neutral atom in a grounded state.
When their electron configurations are added to the table (Figure 6.29), we also see a periodic recurrence of similar electron configurations in the outer shells of these elements. Because they are in the outer shells of an atom, valence electrons play the most important role in chemical reactions. . Aluminum dication loses two electrons Al 2 .

The Electron: Crash Course Chemistry #5. Video 2.6.2 2.6. 2: An overview of the role of orbitals in electron configurations and how to write electron configurations. The relative energy of the subshells determine the order in which atomic orbitals are filled (1 s, 2 s, 2 p, 3 s, 3 p, 4 s, 3 d, 4 p, and so on).electron configuration for aluminum full electron configuration for chlorine The electron configuration for Aluminum is 1s 2 2s 2 2p 6 3s 2 3p 1. The ground state electron configuration is [Ne]3s 2 3p 1. Oxidation States. Aluminum has three oxidation states. The most common one is +3. The other two are +1 and +2. One +3 oxidation state for Aluminum can be found in the compound aluminum oxide, Al 2 O 3. .Electron Configurations are an organized means of documenting the placement of electrons based upon the energy levels and orbitals groupings of the periodic table. The electron configuration for the first 10 elements. H .electron configuration for aluminum Add an electron to the anion electron configuration. For example, the ground state electronic configuration of chlorine is 1s²2s²2p⁶3s²3p⁵. For Cl −, it will be 1s²2s²2p⁶3s²3p⁶. Remove the outermost electrons in the cation, e.g. electron configuration for Mg 2+ will be 1s²2s²2p⁶.
125 Free Spins at Yebo Casino. Exclusive . GamblersLab.com Exclusive Bonus Offer. The EXCLUSIVE BONUS DEALS are unique offers accessible to GamblersLab.com players and can only be claimed by players who visit the casino via our website. Active. This bonus doesn't have an expiration date. Jul 12, 2024. 37 Clicks . .
electron configuration for aluminum|full electron configuration for chlorine